In a Van Gogh Painting, the Flowers Are Changing Color
Scientists have figured out why some of the “Flowers in a blue vase” became discolored over time
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/20120917100004Flowers-in-a-blue-vase-web.jpg)
Since Vincent van Gogh painted “Flowers in a blue vase” in 1887, some of the bouquet’s bright yellow blossoms have turned an orange-gray. Conservators first noticed a gray crust on the cadmium yellow paint in 2009 and were puzzled by the discoloration. But, a group of scientists, in a study to be published in the journal Analytical Chemistry, has determined the culprit: Varnish.
Apparently, sometime in the early 1900s a varnish was applied to the painting with the intention of protecting it. “Varnish can become brown with age and thus can give all colors a more dark tone,” Koen Janssens, a researcher at the University of Antwerp in Belgium, told LiveScience. But the van Gogh discoloration was different.
Painting conservators at the Kröller-Müller Museum in the Netherlands, where the painting is housed, tried to remove the varnish to reveal the painting’s true colors, as is often done. But the varnish and the cadmium yellow paint were inseparable. When the varnish lifted, so too did the mysterious gray crust.
The museum’s experts sent two tiny paint chips, less than a millimeter in size, to Janssens, a chemist and an expert in using X-rays to analyze pigments in oil paintings. He and his colleagues took the samples, fixed in Plexiglass plates, to the European Synchrotron Radiation Facility in Grenoble, France, and Deutsches Elektronen-Synchrotron in Hamburg, Germany. Using X-ray beams, they were then able to study the chemical composition of the samples.

The scientists concluded that a chemical reaction had occurred between the cadmium yellow paint and the varnish. As the painting was exposed to ultraviolet and artificial light, photo-oxidation occurred, freeing the cadmium and sulfate ions in the paint. The sulfate ions then reacted with lead ions in the varnish, which must have had a lead-based drying agent in it. As a result, anglesite, or PbSO4, formed. The cadmium ions also created a layer of cadmium oxalate (CdC2O4). The crusty orange-gray film over some of van Gogh’s yellow flowers is a combination of these two compounds.
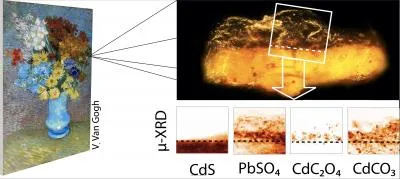
This analysis is the first to reveal this particular reaction, but that isn’t to say that other varnished paintings containing cadmium yellow paint, a pigment introduced during van Gogh’s time, aren’t similarly affected. Ella Hendriks, head of conservation at the Van Gogh Museum in Amsterdam, complimented the way the art and science worlds came together to make this discovery.
“This study on the deterioration of cadmium yellow is an excellent example of how collaboration between scientists and conservators can help to improve our understanding of the condition of van Gogh’s paintings and lead to better preservation of his works,” Hendriks said in a press release. “Many of van Gogh’s French period paintings have been inappropriately varnished in the past, and removal of these non-original varnish layers is one of the challenges facing conservators on a worldwide basis today. The type of information provided by Janssens and his team is vital to support the difficult decisions that conservators often have to make regarding such complex cleaning treatments.”
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/megan.png)
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/megan.png)