Scientists Get Best View Yet of the Structure of Glass
The amorphous solid holds many mysteries, but a new study using a high-powered microscope shows that atoms in glass are organized into distorted shapes
![]()

If glass isn’t a solid or a liquid, then what is it? Photo by Flickr user -Kenzie-
A glass of merlot may make the world look rosy, but it can also be a source of frustration for a physicist. The wine pours, splashes and swirls, yet the glass remains stiff as a solid vessel. Zoom in on the merlot and you’ll see molecules held close together but moving about with no fixed position. Zoom in on the wine glass and you’ll also see this disordered arrangement, but no movement.
On an atomic level, the two forms of matter look the same. Even though a glass is frozen solid, it lacks the rigid crystalline structure found in, say, ice cubes.
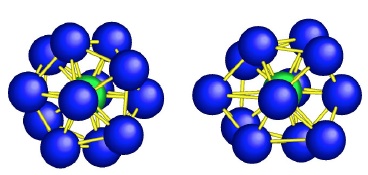
Scientists studying glass observed distorted versions of icosahedrons (icosahedron on left, distorted version on right). Image via Science/Chen and Kotani
Though artisans have been making glass for millennia and scientists have been studying its structure for decades, until now there has been no clear experimental evidence to confirm what prevents liquids that form glasses from crystallizing. In a new paper published online in Science, a team of Japanese researchers used a high-powered electron diffraction microscope to view glass at the tiniest scales yet. At such high resolution they saw what looks to be a basic unit of some glasses–atoms packed in a distorted version of an icosahedron, a three dimensional shape with 20 faces.
With sophisticated geometric tools, the team characterized those distortions, reporting in the paper that they allow the system to “retain dense atomic packing and a low energy state.” Certain arrangements of atoms, the researchers conclude, are the very essence of glassiness because they interfere with the development of a well-organized crystal.
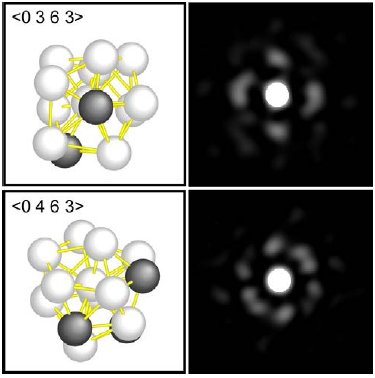
Multiple views of microscopic images of atoms within glass (right) allowed researchers to diagram the level of distortion of the specific icosahedrons that organized the atoms (left). Image via Science/Chen and Kotani
Though the researchers were studying a glass made of zirconium and platinum, not your average windowpane, the results may hold for glasses more broadly. By understanding the ways atoms organize, material scientists can find ways to make new glasses and manipulate the ones they’ve got.
But glass is far from figured out. While the study explains why some liquids form glasses instead of crystallizing, it doesn’t explain why these liquids can become sluggish enough to be solid, says Duke University chemist Patrick Charbonneau. A large community of scientists have been attempting to resolve the sluggishness since the 1980s, but they can’t agree on the solution and they even argue about the best approach.
One popular strategy takes a step back to try to understand how atoms fill a given space. It treats the atoms in glass as hard spheres packed together. Simple, right? “There is no quantum mechanics, there is no string theory, you don’t have to invoke outer space,” Charbonneau says. And yet even studying glass in this way has proven incredibly difficult because of the complications that come with figuring out what positions so many particles could occupy. On top of the inherent challenge of describing the arrangement of the spheres, the approach is a simplification and it is not clear how relevant it would be for real-world glasses.
Still, Charbonneau appears energized when he talks about such research problems. His glass of merlot is half full, because he believes the last few years have brought immense progress. Scientists, he says, have become more creative in asking questions about glass. Charbonneau’s own research simulates glass in higher dimensions, findings that could have important implications for the degree of disorder in three-dimensional glass. Other researchers are considering what would happen if you immobilized some particles in a supercooled liquid, hoping to illuminate how such liquids achieve a glassy state. Still more are considering atoms in glass as entities that can move on their own, sort of like biological cells. All of these efforts are trying to determine the types of interactions that contribute to the formation of glass, so that scientists will recognize a really good sluggishness theory when they see it.
Despite all this talk about movement, don’t expect your wine glass to flow in any visible way anytime soon. This glass “will last longer than the timescale of the universe,” Charbonneau says. Claims that the stained glass in medieval cathedrals is thicker at the bottom because glass flows are bunk. But exactly why it doesn’t flow still remains a mystery.